Medical Devices in general are classified into Class I, II, and III based on the risk and criticality of the indication. As a Class I and Class II Medical Device Manufacturer we understand the intricacies of the process involved.
Here, we shall unfold the basic differences in manufacturing process involved in Class I and Class II Medical Devices. Medical Devices falling in Class I and Class II are exempt from the premarket approval (510k) and GMP requirements as they are considered to be minimally invasive.
Class I Medical Devices
Class I Medical devices are generally meant for primary care. Enema kits, elastic bandages, manual stethoscopes, and bedpans are few common examples of medical products under Class I. For Class I Medical devices registration & listing, Pre-Market Notification, GMP, Record & Report keeping, misbranding, and adulteration of the product are kept into consideration.
Class II Medical Devices
Class II Medical Devices on the other hand are to be regulated according to special controls of safety and effectiveness. The key special controls for the Class II Medical Devices are post-market surveillance, special labeling requirements, patient registries, and pre-market data requirements.

Understanding 510(k) Pre-Market Notification for Class II Medical Devices
The devices falling under Class II must be substantially equivalent to the existing legally marketed device (21 CFR 807.92(a)(3)). When the device matches with the existing substantial equivalence on all grounds then it does not need a PMA (Pre-Market approval).
The 510k clearance is done based on information provided by the FDA. The 510k approval decision is made within 90 days of the application. The maximum time for approval is up to 5 Months. Approximately 80% of the devices comply with existing requirements and match the substantial equivalence requirement submitted by the FDA.
Johari as Leading Class II Medical Device Manufacturer
Johari Digital Healthcare Ltd. is one of the leading class II medical device manufacturer, holds 40+ years of Medical Device Manufacturing experience.
As a globally certified MDSAP and cGMP certified Class II Medical Device Contract Manufacturer, Johari caters to its global clients from a 65,000 square foot, state of the art medical device manufacturing facility in India along with an R&D Center in Europe. Johari’s versatile product development and manufacturing portfolio includes innovative life science products, diagnostic devices, and therapeutic devices specifically Class I and Class II Medical Devices.
Class II Medical Device Manufacturing comprises of several phases. At Johari we ensure keeping eye on every step of the way to make quality device in compliance with existing standards and regulations.
Initial Phase
The initial phase of any class of Medical Device Manufacturing is conceptualization. The process of Conceptualization involves the evaluation of a rough idea on multiple grounds of feasibility testing, competitive analysis, scalability test, and cost structuring. The process also involves setting up the boundaries where the product is to be marketed. Now that you have a concept with you, planning comes into the picture. To go ahead, you need to put a method to each of your portions to make a successful and scalable product. Each step of the process is strategically planned to ensure competitive cost, quality raw material and proper equipment & resource allocation.
Mass Manufacturing or Volume Production
Johari provides Contract Manufacturing solutions to global MedTech giants as well as innovative start-ups. The facility is equipped with advanced capabilities to manufacture complex to high-volume products.
Once prototype is finalized, finally the mass manufacturing begins and proper testing is done at each step of manufacturing. For instance, in the case of Class, I and Class II Medical Device products made at the Johari Manufacturing line, there are tests involved at the PCB Soldering stage (AOI inspection), Assembly stage, and post-assembly stage (Burn Test, Leakage Test).
We have added value at every stage of the manufacturing process:
- Combining advanced manufacturing techniques with Lean Six Sigma principle
- Safeguarding documentation to ensure control over raw materials
- cGMP compliant manufacturing facility
- Optimizing cost
Electronic Assembly and SMT
While printed circuit board assembly (PCBA) is one of our core expertise, we also provide services for assembling and manufacturing sub electronic systems. Our support includes:
- 01005 components, fine pitch and high count BGAs, chip on board, fiber optics, RF microelectronics, and press fit connectors.
- Hybrid processes (tin-lead and lead-free), pin through hole, wave & selective soldering, double and single-sided reflow, wide body and backplanes.
- Quick turn prototype assembly, RoHS compliance certification, conformal & parylene coating.
- Comprehensive electrical testing and test system development for boundary scan, in-circuit test (ICT), functional test and burn in test (BIT).
Variety is our forte!
Types of PCB we can assemble & design
1. Flexible PCB
The wearable device uses Flexible PCB which is easy to mold and fit in a smaller frame. Usage of Flex PCBs ensures an ergonomic user-friendly device. iNishCalm is a sleep-inducing wearable device that uses a flexible PCB for a comfortable application.
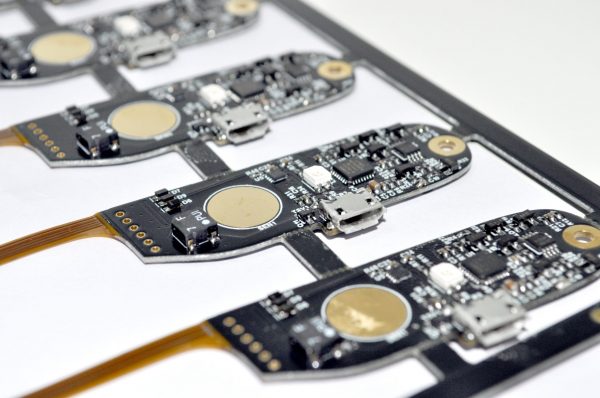
2. Rigid PCB
PCB’s play an important role in the electronic treatment instruments. These play an important role in overall functionality, power source management and timespan required for servicing. Conventionally, there are two types of PCBs including Single Layered PCB and Multilayered PCB. Each PCB differs from the other in terms of functionality and efficiency it delivers in the equipment being produced. A multilayer PCB is highly advanced form of PCB where component space and track space are reduced. At Johari, we design single layered to multi layered PCBs. In our 40 years of experience, we have handled numerous complex projects efficiently. Therefore, our experts can assist you with relevant challenges in PCB design too.
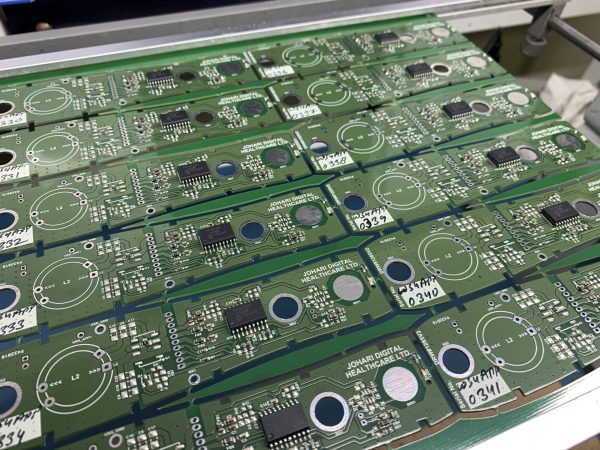
Types of Enclosures
1. Injection Molding Enclosures
Injection Molding is used for creating light weight enclosures in Medical Devices. As an experienced contract manufacturer of medical devices, we understand the contemporary technologies and requirements of the medical industry, to fulfill the shifting demands we work on silicone, propylene, polycarbonate and polyethylene enclosures that provide aesthetic appeal and sturdiness.
2. Sheet Metal Enclosures
Sheet Metal is a very popular enclosure technique for sturdy enclosure formation. We have been providing sturdy sheet metal enclosures over ages.
End-to-End Test Solutions
The best brand world over, in some of the biggest industries, trust Johari to develop meticulous and well-integrated strategies for a variety of testing requirements.
- ICT (In Circuit Test)
- Functional testing
- Test simulation
- Automatic optical inspection
- Burn-in
- Environmental stress
- Packaging tests
With our experience, capabilities, and skill set we are ready to explore expanding horizons in the manufacturing of Point of Care (POC) Medical Devices. Get in touch for Class I and Class II Medical Device Manufacturing.



I need to have meeting with Johari Digital
Hi Nitin,
Thanks for reaching out for Medical device manufacturing. Our team will get back to you soon.
I am interested in creating a class II medical device fibroblast machine. its electrical/high frequency device
Our Team will get back to you soon.