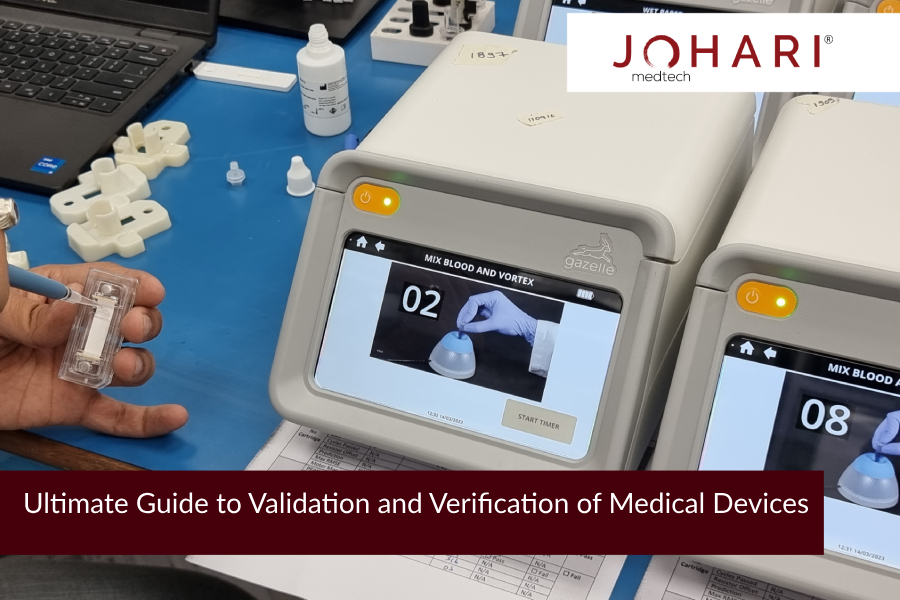
Verification and Validation of Medical Devices is a process requiring continual analysis and development. Often Medical Device Companies have a common panel for the diversity of regulatory and quality-related pursuits. But, it is extremely important to have clarity on who does what! Within the Regulatory & Quality Management System, its imperative to have separate teams for verification and validation. Let’s explore each aspect separately to understand Validation & Verification appropriately.
What is Verification of Medical Devices?
- Verification of Medical devices refers to the process of checking whether all the requirements pertaining to a specific project are met
- The process is necessary for finding out issues related to raw materials, components, parts, and others at the early stage of the development cycle
- The verification systems like software architecture, specifications, high-level design, and database design are verified
- The process involves static testing techniques common to every project
- This is an internal process and does not require external validation from users
What is the Validation of Medical Devices?
- This involves checking whether the end product meets the end-user requirements
- Essential in finding out issues that the verification process cannot identify
- Validation is done for actual manufactured products
- The process of validation comes later in the process
- The process has dynamic testing and mostly it is product-specific
- This is an external process that requires acceptance at the user’s end
On what aspects do Verification & Validation apply?
The verification & validation apply to design, software, product, or process.
Steps of Medical Device Verification
The verification process in general has many tests and trials in place. Verification includes ensuring that your design outputs meet the design inputs. The design verification process is divided into three basic phases:
- Testing
- Inspections
- and analyses
What things to keep in mind while doing validation?
1. Is your device serving the intended purpose?
The primary verification must include steps to ensure the device is performing its assigned function effectively. To understand it better, assume that you’re designing a catheter. Now how much liquid that catheter will have and how fast it will move? This needs to be carefully verified.
2. Understanding the use environment of the device!
The device’s use environment must be carefully evaluated for where it is to be used. For instance, a device designed for use at home cannot be a very big bulky machine. It has to be compact, and sleek and should give accurate results.
3. Keep things concise, clear, crisp, and actionable
Keeping everything documented and actionable helps you stay at pace. Your team requires flow and re-work and too many errors can make everyone’s high-spirited efforts steep low.
To ensure this doesn’t happen, dot down the plan on paper instead of just verbally sharing cues.
4. Design inputs that are testable
Design inputs must be traceable via multi-level testing. The process, in the end, should be able to confirm the intended use. Once the verification is complete you can proceed to the next steps of manufacturing. Verification at the primary stage ensures that when your product enters the mass manufacturing stage you don’t have any major loopholes in the process.
What things to keep in mind while verifying a medical device?
Medical Device Validation has two parts in general.
Medical device manufacturing process Validation:
Medical Device process validation by US FDA is defined in the following way: “Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.”
Medical Device Product Validation:
Medical device product validation is divided into four segments including:
- Prospective Validation: The prospective validation performed before the actual mass manufacturing begins is referred to as prospective validation.
- Concurrent Validation: Concurrent validation is performed on the first three batches at the mass production level.
- Retrospective Validation: Retrospective validation includes analysis of the historical data to ensure the process adopted gives an appropriate quality product.
- Revalidation: The original activities of the validation are performed over & over to ensure consistent results.
Steps of the Process Validation Protocol
1. Identifying the processes to be validated
2. Tracing the medical devices manufactured with these processes
3. Establishing acceptability criteria of the validation results
4. Identifying the operators and equipment to be used in the process
At Johari Digital Healthcare Ltd., we ensure the Verification & Validation of products & processes. Sign up with Johari Digital Healthcare Ltd. for seamless & regulatory-compliant manufacturing services.