Medical Device Regulatory Consulting Services
We offer strategic guidance to our clients at every stage of the product development process. Our regulatory experts have extensive experience in multiple regulatory jurisdictions, including FDA, MDSAP (Australia, Brazil, Canada, Japan, United States), EU MDR, CDSCO and more. We assist in go-to-market of your medical device in any territory across the world.

Go-To-Market Assistance
Regulatory Support :
We offer end-to-end guidance to help you take your product to market. At every stage of your project we assist you in meeting regulatory requirements.
Respect for IP Protection :
We take special care to protect your intellectual property at every step of the way. We follow stringent documentation process to maintain credibility.
Post Market Surveillance :
We value feedback and resolve complaints at the earliest to assure flexibility and improvement in our products & services.
Quality Assurance
We follow (PDCA) Plan, Do, Check, and Act cycle to ensure national and international regulatory compliance.Our key quality assurance measures include quality audits, SOP alignment, tool analysis and staff training.

Quality Control
We perform quality control at every stage of the product lifecycle :
- CAPA management
- Gage R&R analysis
- Statistical process control (SPC)
- Design control
- Lean Six Sigma protocol
- Regular supplier audits & gap analysis
- Process validation
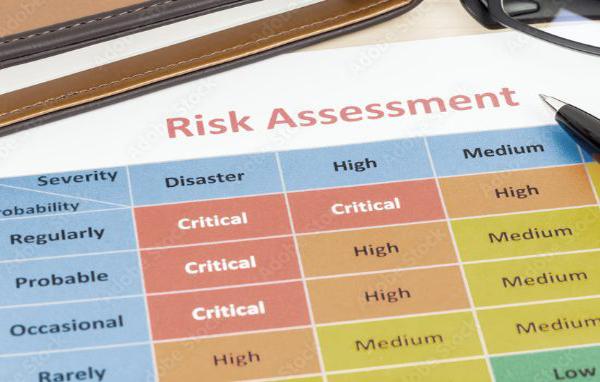
Risk Management
We identify, analyze, and mitigate risks associated with Class I and Class II medical devices. We ensure:
- ISO 14971:2019 implementation
- FMEA
- Health Hazard Evaluation
- Supplier authorisation and qualification over parameters of quality,timeline and efficiency

Pre-dispatch Inspection (PDI)
We ensure the products are packaged well for hassle free shipping & safe
delivery. Our PDI inspection process involve:
- Verification of final goods
- Labelling & packaging control
- UDI verification
DHR/DMR
- We ensure traceability through strong documentation to provide support in case of on-ground failure
- We also maintain DHR and DMR record up to 10 years for long term use

100% Focus on medical device compliance with proven expertise in major categories

Physiotherapy
Aesthetics

Wellness

Life Science

Neuro Rehab

Diagnostic

Beauty
Our Certifications










