A simple purchase decision for a regular use commodity is often influenced by the information printed on the product label. A Medical Device or Product is a high-risk commodity. For such products or devices labeling & packaging plays a crucial role in the final purchase decisions of the buyer.
What does Medical Device Labeling exactly mean?
Medical Device Labeling refers to information printed on the device including symbols, instructions, warnings, control information and more. Labeling of the device can be done by coding, printing, machining, molding and so on. The printed symbols or instructions display the primary information which is absolutely necessary for the user.
Why is Labeling an important part of the Medical Device Development process?
To understand better, consider a fictional labeling malfunction case.
Case:
Suppose a developer is making an insulin delivery device. The labeling indicates the dosage (ml) of insulin to administer. An error occurred at the labeling stage of the Medical Device Development. The dosage value in ml was misquoted on the device package.
Impact
A minor error in the labelling led to millions of wrong dosage administration to patients. The consequences of wrong dosage were severe allergies and some even had to encounter fatal conditions.
Inference
This case reveals the importance labeling holds for any medical device.
Importance of Medical Device Labeling in Class II Medical Devices
Class II Medical Devices include Electronic Physiotherapy and other Non-Invasive Therapeutic Devices. The labeling of these devices indicates specific indicated use, handling methods and various other important details about the device. An example of the same is attached below.
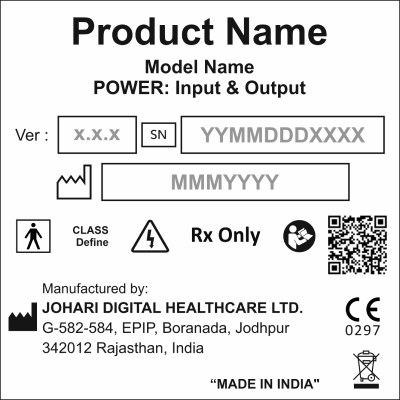
Why Label your Class II Electronic Medical Device?
- FDA Requirements for sale & distribution
- Safety Concerns like:
- Shock
- Fatal injuries
- Biocompatibility issues [Allergies]
- Misuse (Unindicated use)
- Mishandlings of the Device
- Details of Manufacturer/Developer Country
Do’s & Don’ts of Medical Device Labeling
Do’s of Medical Device Labeling
Labeling is a very vivid and vast arena to explore. It can be overwhelming to follow everything stated. Every country in which the device is to be sold has specific labeling & packaging requirements. It’s important to check labeling requirements of each country specifically before taking the product into commercialization.
Sticking to several basic thumb rules can protect you from delays due to labeling issues at the later stage of development.
- Include labeling design at the early stage of the design planning
- Keep the device unique by make, model number, date of manufacture, and batch or serial number
- Include the contact information of the manufacturer/distributor
- Include the box contents with everything inside
- Insert warnings & cautions for the device
- Insert appropriate symbols specific to the device
- Choose the labeling material which remains legible for a long time period
- Include the regulatory teams along with those responsible for Labeling to ensure fast clearance and approval of the new medical device
Don’ts of Medical Device Labeling
- Do not include anything contradictory to intended use
- Marketing statement with no support data
- Usage of the certifying body (e.g., CSA, ETL, UL) artwork on the product label
- Altering the labeling post receiving device clearance
Conclusion
Labeling plays an extremely crucial role in communicating correct usage, ensuring correct identification, communicating the safety instructions and much more.
At Johari, we ensure 100% attention to every detail to make sure your device reaches the market effectively with minimal overdose and unnecessary time lags.
Sign up with Johari Digital Healthcare for accelerated Go-To-Market of your Medical Device.

