Medical Device Design & Development is a multi-step process involving various resources and massive investments and long timelines.
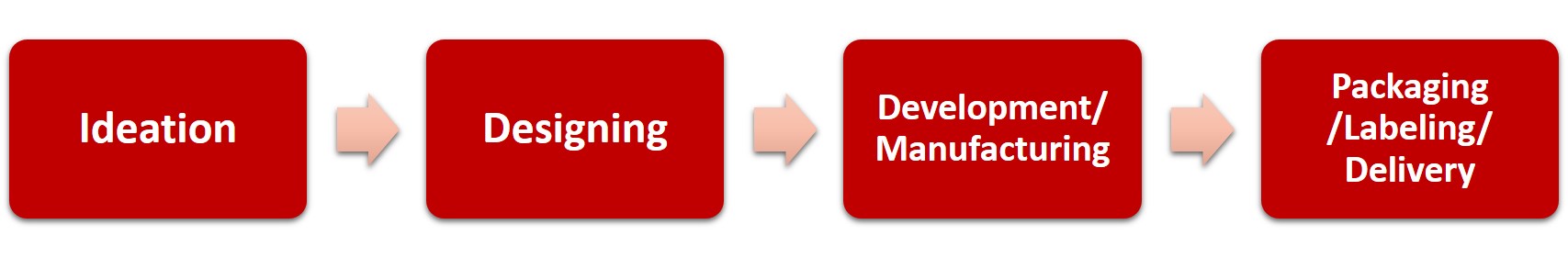
The process has sequential stages of ideation, designing, development, packaging, labeling, and delivery.
Your Medical Device Design must be adaptable to sustain in the competitive landscape for a long time. In the current scenario, when most of the Medical Device OEMs are outsourcing their Medical Device projects a lot depends on the Medical Device Contract Manufacturing partner they choose for mass Manufacturing of their Medical Device Design.
Table of Content
What is Medical Device Design?
Medical device design is the first step towards creating a medical device for the specific intended use (in compliance with US FDA regulatory norms). The product design must serve a unique purpose, delivering something more than already existing versions in the market.
The process of Medical Device Designing involves immense brainstorming and deep analysis to find out the new product’s standing in the market. Once the intended impact and competitive landscape are well understood, you are ready to move ahead. Every stage of the design process is done keeping three things in mind:
- Market need
- Intended impact
- Safety & comfort of the end-user
Missing out on any of these focus areas can lead to delays, significant financial loss, and product recalls.
Phases of Medical Device Design
Your medical device design phase involves many stages such as Industrial design, Mechanical design, software design, and electronic design.
Here, each of the stage is explained briefly:
Medical Device Industrial Design:
Industrial Design is the process of designing products such that the user experience is not compromised at any level. Industrial design in medical device manufacturing is necessary for multiple reasons including:
- Making the devices aesthetically appealing without compromising usability and application.
- Cost-optimized manufacturing
- Ensuring the safety of Medical Devices being manufactured
- Manifesting In-process quick design changes
Medical Device Electronics Design:
The new-age medical devices must have a compact electronic design to enable more efficient Medical Devices. The electronic design eventually decides the power consumption of the device.
Medical Device Software Design:
Software is the backbone of the Medical Device. With most medical devices adopting a connected and data-driven approach, the software has become a highly regulated and most important part of medical devices. With evolving trends in medical software design, it has become utterly important to regulate software in medical devices.
Medical Device Design Control Regulations
Design control is a holistic process of making the device comply with end-user needs. The process involves analyzing the root causes for the failure of products.
- User Needs
- Design Input
- Design Process
- Design Output
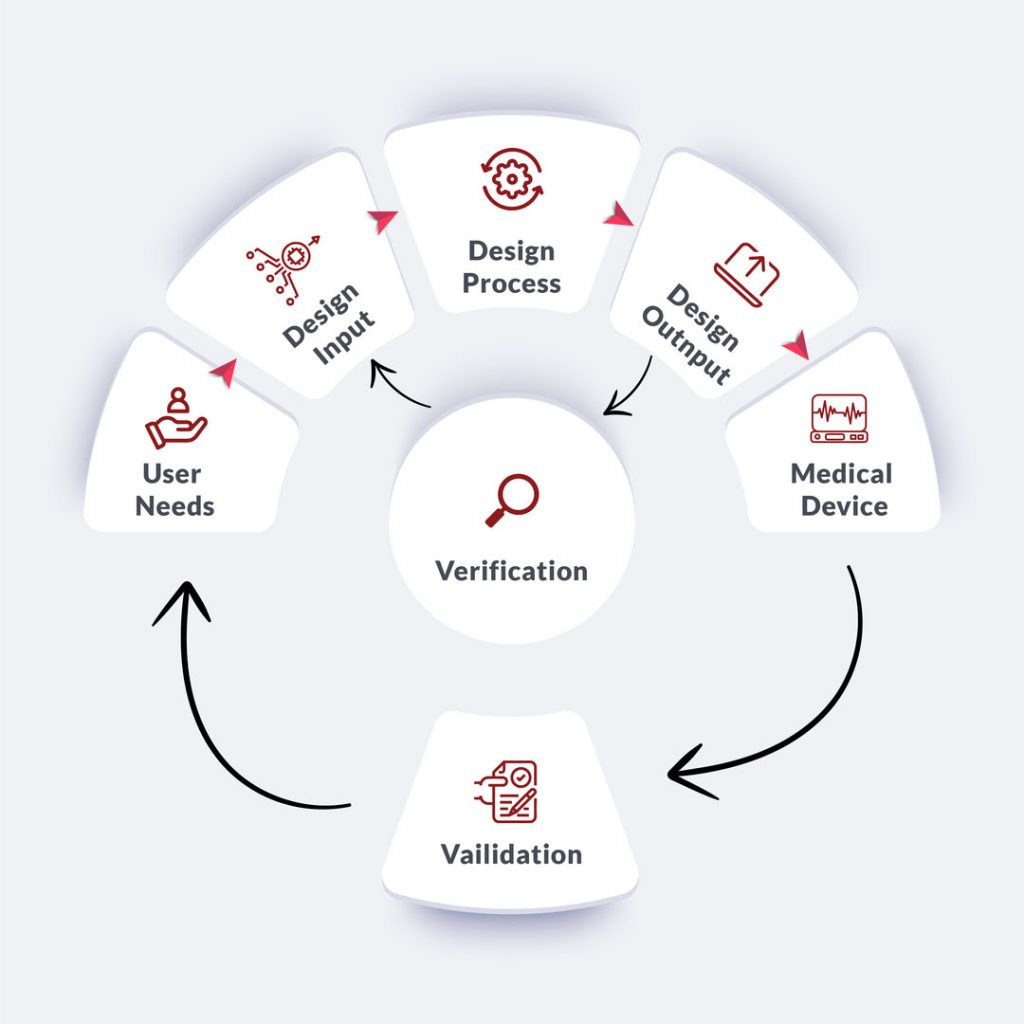
#1. User Needs
The market feedback received is analysed to make modifications according to the market requirement.
#2. Design Input
The process of modifying the design as per the requirements received is the next step in the process.
#3. Design Process
Once the process is known, the design input is converted into high-level specifications. The high-level specifications are then incorporated as features in Medical Devices.
#4. Design Output / Verification process
The process involves transferring to the production facility for mass manufacturing. Design control regulation mandates the process of maintaining DHF (Design History File). The design history file must be traceable and accessible to all team members.
What is Medical Device Development Process?
If you are planning to develop your medical device, these are the multiple stages you need to go through:
- Research and Conceptualization of your Medical Device
- Develop a Prototype
- Development & Manufacturing of your device
- Validation, Testing & Packaging
#1. Research & Conceptualization:
Generally, clients approach the contract manufacturer with an idea or specific modification requirement. At the Medical Device contract manufacturer’s end, the major task is to conduct a 3600 evaluation of the requirements and come up with a relevant concept to solve the problem. Also, it’s not always necessary that there is always a problem to solve, sometimes the client may not be sure about where to begin from? In such a scenario, the contract manufacturer with their design & engineering team has a crucial role to conceptualize the design for scaling production.
#2. Medical Device Prototyping:
When you finalize the medical device design phase, it is transferred for prototyping. Prototyping involves putting the design into a proper 3D shape. Multiple iterations are made to check the user efficiency of the design. Once the design is finalized and the prototype stage is complete it can be transferred ahead for mass manufacturing.
#3. Manufacturing of Medical Devices:
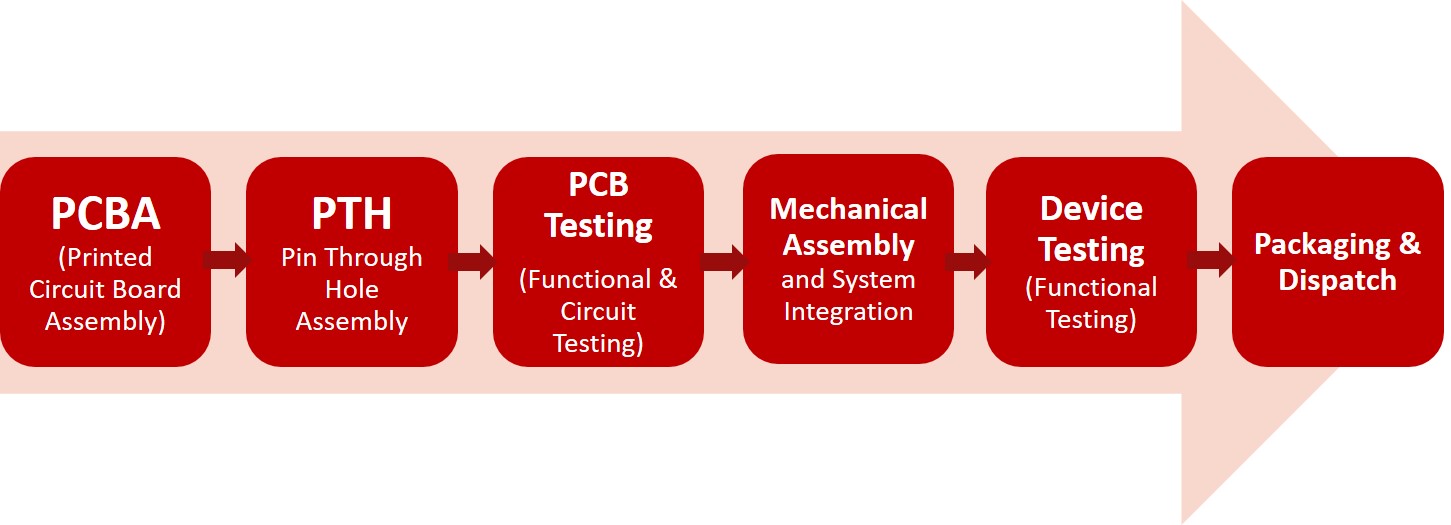
The manufacturing of medical devices refers to a stage when the device enters the scaling phase. In an Electronic Medical Device Manufacturing facility, the manufacturing process is highly controlled to maintain compliance and efficiency. The QA team works closely to continually monitor the products and processes for enhancements and corrections.
The Process of Electronic Medical Device Manufacturing includes:
Some of the key medical devices which are generally manufactured in an Electronic Medical Device Manufacturing company include:
- Cryotherapy Devices
- Arthroscopy & orthopedic surgery support systems
- Battery-Powered Devices & battery changing
- Dermatology Systems
- Doppler ultrasound devices
- Diagnostic carts
- Electronic tissue ablation device
- Endoscopy devices
- Hydrothermal ablation tools
- Vision testing systems
- Cryotherapy Devices
- Fluid Management & monitoring systems
#4. Validation, Testing & Packaging
The testing process post PCBA involves Burn Test, Leakage test, and so on to completely eliminate the chances of error during usage in the end-user environment. Once the testing stage is crossed the product is packaged in compliance with the guidelines issued by FDA.
Benefits of Medical Device Contract Manufacturing and role of Quality Assurance
An Electronic Medical device contract manufacturing services company is the one to which OEMs outsource a part of the manufacturing services to optimize the cost and efficiency. Recently, with the rising global demand for affordable healthcare devices, many big MedTech giants are exploring the outsourcing option to keep things balanced between quality and quantity. Outsourcing Medical Device Manufacturing to a Medical Device Contract Manufacturing partner helps brands to focus on:
- Research and Development
- Branding
Medical device Contract Manufacturing can have a wide range of services covered under its umbrella depending on the client’s requirement. Contract Manufacturers are highly flexible in the way they operate with their clients. Experienced contract manufacturers are instinctive in understanding the client’s requirements. To make it happen they work closely throughout the product lifecycle to surpass the client’s expectations. Medical Device Contract manufacturers work with multiple clients and, this can add value to your project. The skillset and learning can be utilized for your product to make it more robust and innovative.
The Contract manufacturing services cover the following steps along with Quality Assurance involved at every step of the way:
- Research & Conceptualization [QA]
- Prototyping & [QA]
- Manufacturing & [QA]
- Testing & Packaging [QA]
Quality Assurance:
Quality assurance is the process of maintaining quality at every step of the process. From day one of conceptualization, the process of manufacturing is strictly monitored by the Quality assurance experts. The Quality assurance process involves Risk analysis and mitigation measures to substantially reduce the chances of failure post-market entry of the product. QA at all levels also ensures the product complies with all regulatory standards. Complying with all the regulatory norms ensures fast PMA and helps in pitching the product in desired markets. The process involves maintaining the DHR and DMR of the product to trace back the parts in the distant future, in case of any error.
What is a Design History File (DHR)?
A design history File is a formal document with all the minor and major change details. The document also contains the procedures followed and components used in the device. The concerned accountable person details are also enclosed in the document. The file has two core purposes to serve:
- To enable traceability in case of any error.
- To trace the modifications done in the past and upgrade accordingly. DHR can have many other roles along with the purposes mentioned above.
Risk Analysis: How to ensure minimal product error and market re-call chances?
The FMEA (Failure mode effect analysis) evaluation ensures timely identification, evaluation, and mitigation of the risks that might affect the manufacturing process.
RPN Number Analysis
RPN number is assigned to all the risks identified and, priority is decided based on the level of impact it causes. It’s not mandatory to eliminate all the risks involved as mitigating each of the risks may also impact the design and functionality of the medical device. A proper mitigation plan must be implemented for eliminating the risks that might lead to product recalls or any major damage. In case when there is nothing major at stake, the risk might be added as a precautionary note along with the product.
Supply Chain Management of Medical Devices
All major OEMs in Medical Device Industry are looking for trusted outsourcing the manufacturing partner to optimize cost. Once the packaging and labeling are done it’s time for your product to reach its destination. Many contract manufacturers lack enough networks to make cost-optimized shipments.
At Johari Digital Healthcare, we have 400+ vendors across the globe. Our wide range of connected logistic services, smart operations, cost-optimized delivery, and timely communication to clients ensures customer delight.
Johari Digital Healthcare is a 40+ years organization with services in multiple industries including life sciences, biotechnology, physiotherapy, aesthetics, and pain management. We are excellent at providing an extra step with every OEM Medical Device project. Being our partner brings you more than business. Join the Johari community for seamless Medical Device Manufacturing Services.