What is an Electromechanical Medical Device?
An electromechanical medical device combines electronic and mechanical technology for diagnosis, monitoring, or curing of an ailment or a health condition.
On the basis of risk associated and invasiveness of the device, US FDA classifies Non Invasive electromechanical Medical Devices in two categories.
Class I:
Class I Medical Devices comprise 47% of the medical devices. Class I products are simple, low-risk, and do not pose any significant risk. The time to market of these devices is extremely fast.
Class II:
Class II Medical Devices on the other hand comprise 43% of the devices. These have slightly higher ratio of risk associated with each of the devices.
Electromechanical Medical Devices
Electromechanical Devices in Johari digital portfolio:
- Physiotherapy Devices
- Life Science Devices
- Lab Equipment
- Cardiac Monitoring Devices
- Diabetes Monitoring Devices
- Diagnostic and Radiation Devices
- Aesthetic Devices
- In-vitro Diagnostic Devices
Electromechanical Medical Device Design & Development
There are numerous steps in the manufacturing and development of Electromechanical devices. Here we highlight crucial aspects involved in the Medical Device Manufacturing of a Lab device.
Case: Designing & Manufacturing DNA, RNA, and Protein lysis device
Industrial Design
Aesthetic enhancement: In the successive generation of the device, we improved the enclosure aesthetics & incorporated tridimensional motion to overcome the limitation of cell disruption & enhance speed. The dome built by Injection Molding provides 100% transparency. The enclosure has been built by an intelligent combination of Aluminum Alloy, Plastic, Reinforced plastic, and sheet metals. The model design has been checked over international standards for safety against the hazardous situation.
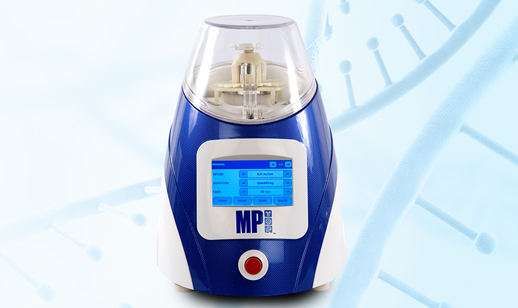
Mechanical Engineering
Noise elimination by Vibration absorbers: The newer version has a dome with efficient Noise proofing technique. Complex machining parts development was done by CNC Machine to optimize cost and design. The vibration absorbers installed in the device are 100% proven to eliminate noise.

Contamination prevention with collection system:
We incorporated a bowl collection system for avoiding contamination. During high-speed rotation often, tubes burst to spill the sample out of place. The newer model efficiently collected the spilled sample and thus prevented contamination.
The current version has a wide area of application in a grind and lyses of an array of sturdy biological samples including yeast, fungi, tissues, bacteria, DNA, RNA, and hair protein. It’s a self-contained system providing high-end disruption & eliminating cross-contamination.
Single platform for multiple tube holders:

The number of Tube Holders has been increased in the current version. Up to 24 samples can be lysed at a time. The modular configuration has detachable tube holders of multiple sizes to process smooth homogenization on a single device platform.
Software Design

Key panel replaced with LCD: The analog design was replaced with a contemporary intuitive User Interface. We expanded the number of operations by replacing the key panel with an LCD touch screen operation. The current version serves responsive, exportable, and smooth operability.
Key applications of Electromechanical Medical Device
Pathology labs, Research labs & Industrial labs require precise & accurate sample fractioning with zero contamination for efficient sampling.
Our Electromechanical Medical Device Capabilities
As a electromechanical medical device contract manufacturer, Johari digital has more than 40 years of expertise with robust manufacturing capabilities and stringent regulatory compliant processes
- Automated PCB Assembly
- Highly Qualified Design & Engineering Team
- Abundant Resources
- US FDA compliant manufacturing facility
- Cost optimized manufacturing
- Strong Supply Chain Network
- Global Presence
Ensuring a Robust Quality Management System for Your Electro-Mechanical Development Process
Working with Johari Digital allows you to develop your device under Johari’s quality system. We are ISO 13485 registered, and our quality systems and practices are compliant with standards IEC 62304, ISO 14971, 93/42/EEC, and IEC 60601. Therefore, Johari’s ISO certifications allow its start-up and established medical device customers to streamline their auditing process and speed up regulatory approvals.
What makes us your trusted Electromechanical Medical Device Manufacturing Partner?
- 80% global clients for CDMO & CMO in Electromechanical Medical Devices
- High bandwidth for diversity of projects
- Skilled Regulatory professionals to assist you in entering Global Market
- Time bound consignment deliveries
- Adding value to your medical device project at any phase of the medical device hardware development process; from design to engineering to testing and more.
Let us show you how we can add immediate value to your medical device development project. Schedule a call today to fast track development of your Electro Mechanical Medical Devices.

